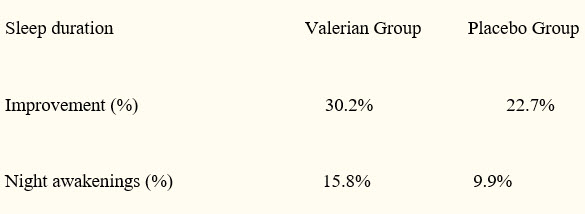Preventing Disease with a Formula for Healthy Sleep
Sleep is required for human life, enabling critical functions such as those involved in cellular regulation and repair, detoxification, immune health, and hormone level modulation.(1-4) Our physiological homeostasis depends on sleep, yet according to the Centers for Disease Control and Prevention (CDC), one in three adults in the United States does not get enough of it.(5) Given the inextricable linkage between sleep and health, the CDC has warned about the health risks of inadequate sleep, and federal and industry dollars continue to fund research that can help elucidate the roles of sleep in disease and quality of life and to provide solutions for those who struggle with poor sleep.
POOR SLEEP PATTERNS CAN BE DETRIMENTAL TO HEALTH
Much of what we know about the benefits of sleep comes from observations and studies of the detrimental effects of sleep deprivation and unhealthy sleep patterns. Accidents are one clear short-term consequence of inadequate sleep. Lack of sleep increases the risk of driving accidents, on-the-job accidents, and accidents in the home.(6-8) However, failing to establish regular, healthy sleep patterns can also lead to chronic disease and disabling symptoms.
Developing and maintaining healthy sleep habits may empower people to reduce their risks of illness and disease. Indeed, poor sleep is associated not only with greater risk for developing a host of health problems, including degenerative diseases, Type 2 diabetes, cardiovascular disease, stroke, and attention deficit hyperactivity disorder (ADHD), but also with a greater risk for suffering debilitating symptoms like migraine headaches and for living a shorter lifepan. (9-16)
Sleep and Degenerative Disease
One degenerative disease for which there is a growing wealth of research into the role of sleep is the neurodegenerative disease, Alzheimer’s. Alzheimer’s disease is the most prevalent cause of dementia in the older population, accounting for 65 to70% of the cases. The formation of amyloid-β (also known as beta amyloid or Aβ) plaques and neurofibrillary tangles are the hallmarks of the disease.
People with healthy sleep habits are at a lower risk for developing Alzheimer’s disease and other forms of dementia.(10) Those at lower risk are those who do not suffer from insomnia and who do not experience sleep disordered breathing (SDB), which includes snoring, sleep apnea, and obstructive sleep apnea. The specific role that sleep plays in protecting against dementia is unclear, but studies have shown that insomnia increases both the production and secretion of amyloid-β, leading to higher levels of amyloid-β in those with insomnia as compared to those with healthy sleep patterns.(17)
Research showing that cerebrospinal levels of amyloid-β and its precursor, amyloid precursor protein (APP), are higher at night suggest that it is during sleep that the brain clears itself of these substances.(18) These findings offer some insight into why sleep seems to protect against neurodegenerative diseases like Alzheimer’s.
Sleep and Type 2 Diabetes
Sleep and Cardiovascular Disease
Sleep and Stroke
Sleep and ADHD
Sleep and the Lifespan
Sleep and Migraine
Migraines and other forms of headache can be associated with a variety of diseases and conditions, but they are also known to be associated with lack of sleep. Though the relationship between sleep and migraine is complex,(19) it is clear that the two often co-occur. Indeed, disturbed sleep is more common in adults and children with migraine than those without migraine, with between 30% and 50% of migraine patients experiencing disturbed sleep or poor sleep quality.(20-23) Further, the severity and prevalence of sleep problems increase proportionally with headache frequency, such that the vast majority of chronic migraineurs (68% to 84%) suffer from insomnia on a near-daily basis.(20)
There is evidence that lack of sleep causes migraines and that, conversely, migraines cause loss of sleep. It is therefore likely that migraineurs with disturbed sleep experience a negative feedback loop where migraines and loss of sleep reinforce one another and relief from either condition becomes harder and harder.(20-22) Nonetheless, restful sleep has been shown to be effective in relieving migraine attacks, strongly suggesting that insufficient sleep causes or exacerbates migraine headaches.
Consistent with this view is the finding that those with migraines are less likely to possess the ability to flexibly adapt their sleep/wake cycles (24) and are thus more likely to become sleep deprived. Even more telling is that lack of sleep is the most commonly reported trigger of headaches.(25,26)
NATURAL ALTERNATIVES FOR SLEEP
Alternative headache and migraine therapies include psychological counseling, biofeedback, and physical therapy, which work by making lifestyle changes. Non-pharmacological treatments for the management of migraines and headaches has a growing field of science to support their use. Biofeedback techniques teach patients to control certain responses of their body to help reduce pain. For example, a patient can learn diaphragmatic breathing, heart rate, muscle tension and how to control temperature to enter a relaxed state, which may bring about better pain control.
Alternative treatments for insomnia and disordered sleep include background music, acupuncture, prayer, deep breathing, meditation, yoga and massage.
Non-pharmacological treatments include natural supplements for sleep which avoids the serious side effects of prescription drugs. Drug-related side effects include kidney damage, ulcers, dependence, addiction, tolerance development requiring higher doses, rebound insomnia, withdrawal symptoms and daytime grogginess. (19, 20, 21)
Another aspect of over-the counter NSAID’s (non-steroidal anti-inflammatory drugs) and prescription drugs is that analgesic over-use can cause chronic headache syndrome, where the drug increases the number of migraine episodes per month. Natural supplements have never been reported to cause this effect. (22, 23)
Ingredients Discussion
Hops extract
The sedative power of Hops extract has long been recognized and is associated withover 30 years of traditional medicinal use in Europe. Recently, it has been suggested that its acids, essential oil, and other constituents, such as xanthohumol, may play important roles in the sedative effect of Hops preparations.(27)
The results of clinical studies suggest that Hops extract may help to improve sleep quality, shorten time to fall asleep, improve sleep brain wave patterns, and improve subjective measures of restfulness after waking.(28-33) One study has provided some clarification on how Hops extract may confer its sleep benefits, suggesting that Hops may improve the ability to fall asleep by reducing anxiety.(34) In this study, pure Hops extract in a non-alcoholic beer (0.00% alcohol, 333 milliliters (ml). containing 0.3% or 100 milligrams (mg) of Hops) was administered to a work-stressed population of healthy female nurses in rotating night shifts to measure the sedative effect of hops on their sleep and wake rhythm patterns.
Overnight sleep parameters were assessed by an actigraph for fourteen days, and the hops extract was given with dinner. The sleep data were compared with subjects’ base data, which had been collected before Hops consumption began.(34)
Actigraphy demonstrated not only an improvement in overall sleep quality but a specific improvement in sleep latency. Specifically, those taking the Hops extract experienced a reduction in sleep latency, falling asleep more quickly. Compared to 21 minutes before using the Hops extract, those taking the Hops extract fell asleep on average within 12 minutes. These individuals also showed reductions in self-reported levels of anxiety.
The effective therapeutic dose of Hops for improved sleep parameters is 60 to 100 mg based on the above-cited human trial. Any sleep formula with less than 60 mg. of Hops extract would have be considered an inadequate dose.(34)
In addition to its ability to aid in sleep, Hops extract has numerous other health benefits, including stimulating the production of antioxidant enzymes, protecting DNA against mutations, protectiing against thrombosis formation, which refers to the formation of blood clots, and protecting against benzopyrene and other dietary carcinogens.(35–37)
Valerian extract
Valerian, Valeriana officinalis, is a perennial herb native to North America, Asia, and Europe and is used for its sedative and hypnotic properties. Multiple preparations are available, and the herb is commonly combined with other herbals, primarily Hops extract Double-blind, placebo-controlled research has demonstrated that adults who take valerian for two weeks judge their sleep as better than those who take placebo. According to study subjects, this higher sleep quality is due to fewer night awakenings and greater sleep duration.(38)
Source: Oxman, A, Flottorp, S, Håvelsrud, K, Fretheim, A. et al. PLoS One. 2007; 2(10): e1040.
The appropriate dosage of Valerian has not been established conclusively but The European Medicines Agency (EMEA) final proposal recommends a single dose of two to three grams (of dried herb) one half to one hour before bedtime with an earlier dose during the evening if necessary. Based on human trial data, a total serving size of 400 to 500 mg of Valerian extract would be considered an adequate dose of Valerian extract for a sleep supplement.
In addition to its positive effects on sleep duration and night awakenings, Valerian also has several other uses. It is used to treat anxiety, depression, menopausal symptoms, and stress and is among the eight most widely used herbal supplements in the world.(39,40)
Zizyphus Jujube extract
The Ziziphus jujuba seed extract, also known by its Chinese name, suanZaorentang, is the herb most frequently used for insomnia in China.(41) In Taiwan, it is the second most prescribed phytomedicine to treat insomnia.(42) The effect of Ziziphus jujube seeds on insomnia is clinically comparable to what is observed with benzodiazepines, the primary medical treatment for insomnia and other sleep disorders in primary care practice. Unlike benzodiazepines, however, Ziziphus has no withdrawal side effects.
Ziziphus spinosa is a varietal of Ziziphus jujube, and its impact on sleep is likely related to its stimulation of the inhibitory neurotransmitter, GABA, and its influence on serotonin receptors in the brain.(41,43) Suanzaorentang may also benefit sleep through sedative-hypnotic effects and its anxiolytic (anti-anxiety) properties, which are comparable to those of diazepam, a benzodiazepine drug.(44) Indeed, research on this substance has shown that, compared to placebo, it improves, to a greater extent, all ratings of sleep quality in insomniac patients.(45) The Zizyphus jujube seed extract effective dose for insomnia is 100 mg to 200 mg. Thus, any amount less than that would be inadequate for achieving the desired effects of relaxation or sleep in any formula. In addition to its use for insomnia, the seed of of Zizyphus jujuba spinosa variety has also been used to treat psychiatric disorders in both Korean and Traditional Chinese Medicine (TCM). Its advantageous impact on sleep could also account for additional neuroprotective characteristics of Zizyphus jujube seed extract that have recently been observed. Specifically, the extract has been shown to mitigate memory loss associated with most neurological diseases and to block amyloid β-induced memory deficits in a mouse model of Alzheimer’s disease.(46)
Glycine
Early research on glycine and its essential role in sleep was published in 1989.(47) Later, in 2008, one of the ways in which glycine aids in sleep was clarified when it was discovered that glycine is responsible for the profound muscle relaxation that occurs during various stages of rapid eye movement (REM) sleep.(48)
The first clinical trial using glycine supplementation found that people who were given glycine before bedtime reported significantly reduced feelings of fatigue the following morning when compared to those who were given placebo. This study employed 19subjects ranging from 24 to 53 years and assessed sleep quality with the St. Mary’s Hospital Sleep Questionnaire (SMHSQ) and the Space Aeromedicine Fatigue Checklist.(49)
In another study, glycine improved subjects’ sleep efficiency,reduced difficulty in falling asleep, and enhanced sleep satisfaction. This study used both objective measures of sleep quality, including polysomnographic tests and the Visual Analog Scale (VAS), a well as subjective measures of sleep, including the SMHSQ. Each measure suggested that daytime sleepiness improved and that glycine helped with overall sleep quality.(50)
A dose of 31 grams of glycine per day has been shown to be associated with no serious side effects.(51)
Glycine has also been shown to improve memory. A 1999 double-blind study showed that compared to younger individuals, middle-aged men had poorer verbal episode memory recall, sustained, focused, or divided attention. However, glycine supplementation significantly improved episodic memory retrieval in both the young and the middle-aged subjects. Unlike other cognitive enhancing supplements, glycine has no stimulant properties or mood effects. (52)
Pyridoxyl-5-Phosphate
Pyridoxal-5-Phosphate, or P5P, is the active metabolite of vitamin B6. There are three forms of vitamin B6 found in the diet or in supplements that are usually found in the form of hydrocholoride salts – pyridoxine, pyridoxamine and pyridoxal.(53) Though to take effect, pyridoxine and pyridoxamine need to be converted to their active B6 form, P5P, age and impaired liver function can hinder this conversion.(54) P5P supplementation bypasses the need for this conversion and allows for more immediate benefits.
Studies have shown that P5P impacts aspects of sleep. For instance, compared to those taking a placebo, college students who took P5P before bed reported higher levels of dream vividness and emotionality. According to the researchers conducting the study, these results may have been due to vitamin B6-induced cortical arousal during periods of REM sleep.(55)
Any dose of pyridoxine ranging from 25 to 100 mg is considered adequate for neurotransmitter production to support sleep.
Vitamin B6 is a co-factor for a host of enzymatic reactions and thus plays a role in a multitude of functions. For instance, adequate levels of B6 are essential for promoting and maintaining mood, pain perception, and keeping inflammation and inflammatory markers, such as c-reactive protein (CRP), at normal levels.(56,57)
Deficiencies in vitamin B6 result in less methylation of the genes that produce nitric oxide, which, in turn, can trigger a migraine.(58) Vitamin B6 supplementation has also been shown to effectively reduce headache severity and the duration of migraine attacks. Given that nitric oxide deficiency can cause artery vasodilation and that vitamin B6 helps to control nitric oxide availability in the cell, it is perhaps not surprising that B6 supplementation can help in overcoming headaches.
Magnesium
Magnesium is involved in over 300 enzyme-related biochemical processes and appears to influence sleep in a variety of ways. Those who are deficient in magnesium are more likely to have abnormal EEG readings during sleep, more nocturnal awakenings, less time spent in stage 5 REM sleep, and self-reports of poor sleep quality.(59)
On the other hand, those taking dietary magnesium supplements are more likely to experience better sleep efficiency, the ability to fall asleep faster, and the ability to stay asleep longer, which may be related to the ability of magnesium to reduce cortisol levels and inflammatory stress and to enhance melatonin levels.(60,61) Magnesium supplementation is associated with reversals in exercise intolerance that are observed in chronically sleep-deprived individuals(62) as well as the restoration of normal EEG patterns during sleep.(63)
Magnesium is involved not only in sleep but in a variety of aspects of health. One important way that magnesium improves health is by reducing CRP levels. CRP is a general indicator of inflammation in the body, and higher levels of CRP are associated with a higher risk of developing degenerative disease.(64,65)
Overall systemic inflammation caused by Magnesium deficiency will lead to sleep disturbances and supplementation with Magnesium lowers key inflammatory cytokine molecules, CRP, TNF- α, and IL-6.
Melatonin
Melatonin is a hormone produced by the pineal gland that helps to control our body’s biorhythms and thereby regulates sleep.(66) It has become one of the most frequently used non-prescription sleep aids. Melatonin helps to promote total sleep time and recovery from jet lag fatigue, and can help balance the circadian rhythm disruption that occurs with rotating shift work.(67) Mounting evidence suggests that melatonin can increase sleep efficiency, reduce the time it takes for people to fall asleep, and increase total sleep duration.(68,69,70) Melatonin levels decline with age, which is believed to contribute to age-related sleep disorders and age-related diseases.(71)
Research conducted at the Massachusetts Institute of Technology (MIT) has shown that a dose of 0.3 mg of melatonin restored sleep in people over the age of 50, allowing those who normally wake up in the latter two thirds of the night to sleep all the way through the night. Interestingly, the study also found that a typical health food store dosage of melatonin is roughly 3 mg and that this dosage is not only less effective in treating insomnia, but it is also associated with potentially serious side effects.(72,73)
In addition to its sleep benefits, melatonin has been shown to be as effective in reducing migraine attack severity, frequency, and duration as the anticonvulsant, sodium valproate, also known as valproic acid.(74) Unlike sodium valproate, however, is better tolerated. It has not been shown to lead to any of the adverse side effects associated with sodium valproate. In addition, a comprehensive meta-analysis was recently performed to assess the safety of efficacy of melatonin in children with sleep and neurodevelopmental disorders and found that melatonin is both safe and effective in these youth populations.(75)
Other benefits of melatonin are that it has potent antioxidant properties,(76) lowers inflammatory markers, and is an immune modulator.(77,78) It also contributes to healthy cardiovascular function,(79) plays a role in eye health,(80) and impacts fat and glucose metabolism.(81) As a powerful free radical scavenger, melatonin can directly remove excess free radicals produced during episodes of oxidative stress and can favorably change antioxidant gene expression.(82)
References
1. Benington JH, Heller HC. Restoration of brain energy metabolism as the function of sleep. Prog Neurobiol. 1995;45(4):347-360.
2. Berger RJ, Phillips NH. Energy conservation and sleep. Behav Brain Res. 1995;69(1-2):65-73.
3. Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373-377. doi:10.1126/science.1241224
4. Siegel JM. Sleep viewed as a state of adaptive inactivity. Nat Rev Neurosci. 2009;10(10):747-753. doi:10.1038/nrn2697
5. HHS. 1 in 3 adults don’t get enough sleep: A good night’s sleep is critical for good health. Centers for Disease Control and Prevention (CDC). https://www.cdc.gov/media/releases/2016/p0215-enough-sleep.html. Published 2016.
6. Akerstedt T, Philip P, Capelli A, Kecklund G. Sleep loss and accidents–work hours, life style, and sleep pathology. Prog Brain Res. 2011;190:169-188. doi:10.1016/B978-0-444-53817-8.00011-6
7. Wade AG. The societal costs of insomnia. Neuropsychiatr Dis Treat. 2010;7:1-18. doi:10.2147/NDT.S15123
8. Leger D, Massuel M-A, Metlaine A. Professional correlates of insomnia. Sleep. 2006;29(2):171-178.
9. Malhotra RK. Neurodegenerative disorders and sleep. Sleep Med Clin. 2018;13(1):63-70. doi:10.1016/j.jsmc.2017.09.006
10. Shi L, Chen S-J, Ma M-Y, et al. Sleep disturbances increase the risk of dementia: A systematic review and meta-analysis. Sleep Med Rev. 2018;40:4-16. doi:10.1016/j.smrv.2017.06.010
11. Kawakami N, Takatsuka N, Shimizu H. Sleep disturbance and onset of type 2 diabetes. Diabetes Care. 2004;27(1):282-283.
12. Bassetti CL. Sleep and stroke. Semin Neurol. 2005;25(1):19-32. doi:10.1055/s-2005-867073
13. Sofi F, Cesari F, Casini A, Macchi C, Abbate R, Gensini GF. Insomnia and risk of cardiovascular disease: a meta-analysis. Eur J Prev Cardiol. 2014;21(1):57-64. doi:10.1177/2047487312460020
14. Um YH, Hong S-C, Jeong J-H. Sleep problems as predictors in attention- hyperactivity disorder: causal mechanisms, consequences and treatment. Clin Psychopharmacol Neurosci. 2017;15(1):9-18. doi:10.9758/cpn.2017.15.1.9
15. Li Y, Zhang X, Winkelman JW, et al. Association between insomnia symptoms and mortality: a prospective study of U.S. men. Circulation. 2014;129(7):737-746. doi:10.1161/CIRCULATIONAHA.113.004500
16. Lin Y-K, Lin G-Y, Lee J-T, et al. Associations between sleep quality and migraine frequency: A cross-sectional case-control study. Medicine (Baltimore). 2016;95(17):e3554. doi:10.1097/MD.0000000000003554
17. Ooms S, Overeem S, Besse K, Rikkert MO, Verbeek M, Claassen JAHR. Effect of 1 night of total sleep deprivation on cerebrospinal fluid beta-amyloid 42 in healthy middle-aged men: a randomized clinical trial. JAMA Neurol. 2014;71(8):971-977. doi:10.1001/jamaneurol.2014.1173
18. Tarasoff-Conway JM, Carare RO, Osorio RS, et al. Clearance systems in the brain-implications for Alzheimer disease. Nat Rev Neurol. 2015;11(8):457-470. doi:10.1038/nrneurol.2015.119
19. Walsh JK, Coulouvrat C, Hajak G, et al. Nighttime insomnia symptoms and perceived health in the America Insomnia Survey (AIS). Sleep. 2011;34(8):997-1011. doi:10.5665/SLEEP.1150
20. Kelman L, Rains JC. Headache and sleep: examination of sleep patterns and complaints in a large clinical sample of migraineurs. Headache. 2005;45(7):904-910. doi:10.1111/j.1526-4610.2005.05159.x
21. Sahota P. Morning headaches in patients with sleep disorders. Sleep Med. 2003;4(5):377.
22. Seidel S, Hartl T, Weber M, et al. Quality of sleep, fatigue and daytime sleepiness in migraine – a controlled study. Cephalalgia. 2009;29(6):662-669. doi:10.1111/j.1468-2982.2008.01784.x
23. Rasmussen BK. Migraine and tension-type headache in a general population: precipitating factors, female hormones, sleep pattern and relation to lifestyle. Pain. 1993;53(1):65-72.
24. van Oosterhout W, van Someren E, Schoonman GG, et al. Chronotypes and circadian timing in migraine. Cephalalgia. 2018;38(4):617-625. doi:10.1177/0333102417698953
25. Spierings EL, Ranke AH, Honkoop PC. Precipitating and aggravating factors of migraine versus tension-type headache. Headache. 2001;41(6):554-558.
26. Kelman L. The triggers or precipitants of the acute migraine attack. Cephalalgia. 2007;27(5):394-402. doi:10.1111/j.1468-2982.2007.01303.x
27. Franco L, Sanchez C, Bravo R, Rodriguez A, Barriga C, Juanez JC. The sedative effects of hops (Humulus lupulus), a component of beer, on the activity/rest rhythm. Acta Physiol Hung. 2012;99(2):133-139. doi:10.1556/APhysiol.99.2012.2.6
28. Ross SM. Sleep disorders: a single dose administration of valerian/hops fluid extract (dormeasan) is found to be effective in improving sleep. Holist Nurs Pract. 2009;23(4):253-256. doi:10.1097/HNP.0b013e3181aed09d
29. Dimpfel W, Suter A. Sleep improving effects of a single dose administration of a valerian/hops fluid extract – a double blind, randomized, placebo-controlled sleep-EEG study in a parallel design using electrohypnograms. Eur J Med Res. 2008;13(5):200-204.
30. Koetter U, Schrader E, Kaufeler R, Brattstrom A. A randomized, double blind, placebo-controlled, prospective clinical study to demonstrate clinical efficacy of a fixed valerian hops extract combination (Ze 91019) in patients suffering from non-organic sleep disorder. Phytother Res. 2007;21(9):847-851. doi:10.1002/ptr.2167
31. Schellenberg R, Sauer S, Abourashed EA, Koetter U, Brattstrom A. The fixed combination of valerian and hops (Ze91019) acts via a central adenosine mechanism. Planta Med. 2004;70(7):594-597. doi:10.1055/s-2004-827180
32. Schmitz M, Jackel M. [Comparative study for assessing quality of life of patients with exogenous sleep disorders (temporary sleep onset and sleep interruption disorders) treated with a hops-valarian preparation and a benzodiazepine drug]. Wien Med Wochenschr. 1998;148(13):291-298.
33. Muller-Limmroth W, Ehrenstein W. [Experimental studies of the effects of Seda-Kneipp on the sleep of sleep disturbed subjects; implications for the treatment of different sleep disturbances (author’s transl)]. Med Klin. 1977;72(25):1119-1125.
34. Franco L, Sanchez C, Bravo R, et al. The sedative effect of non-alcoholic beer in healthy female nurses. PLoS One. 2012;7(7):e37290. doi:10.1371/journal.pone.0037290
35. Dietz BM, Kang Y-H, Liu G, et al. Xanthohumol isolated from Humulus lupulus Inhibits menadione-induced DNA damage through induction of quinone reductase. Chem Res Toxicol. 2005;18(8):1296-1305. doi:10.1021/tx050058x
36. Xin G, Wei Z, Ji C, et al. Xanthohumol isolated from Humulus lupulus prevents thrombosis without increased bleeding risk by inhibiting platelet activation and mtDNA release. Free Radic Biol Med. 2017;108:247-257. doi:10.1016/j.freeradbiomed.2017.02.018
37. Plazar J, Zegura B, Lah TT, Filipic M. Protective effects of xanthohumol against the genotoxicity of benzo(a)pyrene (BaP), 2-amino-3-methylimidazo[4,5-f]quinoline (IQ) and tert-butyl hydroperoxide (t-BOOH) in HepG2 human hepatoma cells. Mutat Res. 2007;632(1-2):1-8. doi:10.1016/j.mrgentox.2007.03.013
38. Oxman AD, Flottorp S, Havelsrud K, et al. A televised, web-based randomised trial of an herbal remedy (valerian) for insomnia. PLoS One. 2007;2(10):e1040. doi:10.1371/journal.pone.0001040
39. Pallesen S, Bjorvatn B, Nordhus IH, Skjerve A. [Valerian as a sleeping aid?]. Tidsskr Nor Laegeforen. 2002;122(30):2857-2859.
40. Morris CA, Avorn J. Internet marketing of herbal products. JAMA. 2003;290(11):1505-1509. doi:10.1001/jama.290.11.1505
41. Ni X, Shergis JL, Guo X, et al. Updated clinical evidence of Chinese herbal medicine for insomnia: a systematic review and meta-analysis of randomized controlled trials. Sleep Med. 2015;16(12):1462-1481. doi:10.1016/j.sleep.2015.08.012
42. Rodriguez Villanueva J, Rodriguez Villanueva L. Experimental and clinical pharmacology of ziziphus jujuba mills. Phytother Res. 2017;31(3):347-365. doi:10.1002/ptr.5759
43. Shergis JL, Ni X, Sarris J, et al. Ziziphus spinosa seeds for insomnia: A review of chemistry and psychopharmacology. Phytomedicine. 2017;34:38-43. doi:10.1016/j.phymed.2017.07.004
44. Hsieh MT, Chen HC, Kao HC, Shibuya T. Suanzaorentang, and anxiolytic Chinese medicine, affects the central adrenergic and serotonergic systems in rats. Proc Natl Sci Counc Repub China B. 1986;10(4):263-268.
45. Chen HC, Hsieh MT. Clinical trial of suanzaorentang in the treatment of insomnia. Clin Ther. 1985;7(3):334-337.
46. Kwon H, Jung IH, Yi JH, et al. The Seed of Zizyphus jujuba var. spinosa Attenuates Alzheimer’s Disease-Associated Hippocampal Synaptic Deficits through BDNF/TrkB Signaling. Biol Pharm Bull. 2017;40(12):2096-2104. doi:10.1248/bpb.b17-00378
47. Chase MH, Soja PJ, Morales FR. Evidence that glycine mediates the postsynaptic potentials that inhibit lumbar motoneurons during the atonia of active sleep. J Neurosci. 1989;9(3):743-751.
48. Soja PJ. Glycine-mediated postsynaptic inhibition is responsible for REM sleep atonia. Sleep. 2008;31(11):1483-1486.
49. Inagawa K et al. Subjective effects of glycine ingestion before bedtime on sleep quality. Sleep Biol Rythm. 2006;4:75-77.
50. Yamadera W et al. Glycine ingestion improves subjective sleep quality in human volunteers, correlating with polysomnographic changes. Sleep Biol Rhythm. 2007;5:126-131.
51. Inagawa K et al. Assessment of acute adverse events of glycine ingestion at a high dose in human volunteers. J Urban Living Heal Assoc. 2006;50:27-32.
52. File SE, Fluck E, Fernandes C. Beneficial effects of glycine (bioglycin) on memory and attention in young and middle-aged adults. J Clin Psychopharmacol. 1999;19(6):506-512.
53. Johansson S, Lindstedt S, Tiselius HG. Metabolic interconversions of different forms of vitamin B6. J Biol Chem. 1974;249(19):6040-6046.
54. Russell RM. Factors in aging that effect the bioavailability of nutrients. J Nutr. 2001;131(4 Suppl):1359S-61S. doi:10.1093/jn/131.4.1359S
55. Ebben M, Lequerica A, Spielman A. Effects of pyridoxine on dreaming: a preliminary study. Percept Mot Skills. 2002;94(1):135-140. doi:10.2466/pms.2002.94.1.135
56. Morris MS, Sakakeeny L, Jacques PF, Picciano MF, Selhub J. Vitamin B-6 intake is inversely related to, and the requirement is affected by, inflammation status. J Nutr. 2010;140(1):103-110. doi:10.3945/jn.109.114397
57. Friso S, Jacques PF, Wilson PW, Rosenberg IH, Selhub J. Low circulating vitamin B(6) is associated with elevation of the inflammation marker C-reactive protein independently of plasma homocysteine levels. Circulation. 2001;103(23):2788-2791.
58. Sadeghi O, Nasiri M, Maghsoudi Z, Pahlavani N, Rezaie M, Askari G. Effects of pyridoxine supplementation on severity, frequency and duration of migraine attacks in migraine patients with aura: A double-blind randomized clinical trial study in Iran. Iran J Neurol. 2015;14(2):74-80.
59. Popoviciu L et al. Clinical and Polysomnographic Researches in Patients with Sleep Disorders Associated with Magnesium Deficiencies. London: John Libbey; 1991.
60. Abbasi B, Kimiagar M, Sadeghniiat K, Shirazi MM, Hedayati M, Rashidkhani B. The effect of magnesium supplementation on primary insomnia in elderly: A double-blind placebo-controlled clinical trial. J Res Med Sci. 2012;17(12):1161-1169.
61. Nielson, FH, Johnson, LK, Zeng H. Magnesium supplementation improves indicators of low magnesium status and inflammatory stress in adults older than 51 years with poor sleep quality. Magesium Res. 2010;23(4):158-168.
62. Tanabe K, Yamamoto A, Suzuki N, et al. Efficacy of oral magnesium administration on decreased exercise tolerance in a state of chronic sleep deprivation. Jpn Circ J. 1998;62(5):341-346.
63. Held K, Antonijevic IA, Kunzel H, et al. Oral Mg(2+) supplementation reverses age-related neuroendocrine and sleep EEG changes in humans. Pharmacopsychiatry. 2002;35(4):135-143. doi:10.1055/s-2002-33195
64. McGeer PL, McGeer EG. Inflammation and the degenerative diseases of aging. Ann N Y Acad Sci. 2004;1035:104-116. doi:10.1196/annals.1332.007
65. Dibaba DT, Xun P, He K. Dietary magnesium intake is inversely associated with serum C-reactive protein levels: meta-analysis and systematic review. Eur J Clin Nutr. 2014;68(4):510-516. doi:10.1038/ejcn.2014.7
66. Wagner J, Wagner ML, Hening WA. Beyond benzodiazepines: alternative pharmacologic agents for the treatment of insomnia. Ann Pharmacother. 1998;32(6):680-691. doi:10.1345/aph.17111
67. Pandi-Perumal SR, Srinivasan V, Spence DW, Cardinali DP. Role of the melatonin system in the control of sleep: therapeutic implications. CNS Drugs. 2007;21(12):995-1018. doi:10.2165/00023210-200721120-00004
68. Pevet P, Challet E. Melatonin: both master clock output and internal time-giver in the circadian clocks network. J Physiol Paris. 2011;105(4-6):170-182. doi:10.1016/j.jphysparis.2011.07.001
69. Rossignol DA, Frye RE. Melatonin in autism spectrum disorders: a systematic review and meta-analysis. Dev Med Child Neurol. 2011;53(9):783-792. doi:10.1111/j.1469-8749.2011.03980.x
70. Brzezinski A, Vangel MG, Wurtman RJ, et al. Effects of exogenous melatonin on sleep: a meta-analysis. Sleep Med Rev. 2005;9(1):41-50. doi:10.1016/j.smrv.2004.06.004
71. Karasek M. Melatonin, human aging, and age-related diseases. Exp Gerontol. 2004;39(11-12):1723-1729. doi:10.1016/j.exger.2004.04.012
72. Zhdanova I V, Wurtman RJ, Regan MM, Taylor JA, Shi JP, Leclair OU. Melatonin treatment for age-related insomnia. J Clin Endocrinol Metab. 2001;86(10):4727-4730. doi:10.1210/jcem.86.10.7901
73. Halber D. Scientists pinpoint dosage of melatonin for insomnia. MIT News. October 2001. http://news.mit.edu/2001/melatonin-1017.
74. Ebrahimi-Monfared M, Sharafkhah M, Abdolrazaghnejad A, Mohammadbeigi A, Faraji F. Use of melatonin versus valproic acid in prophylaxis of migraine patients: A double-blind randomized clinical trial. Restor Neurol Neurosci. 2017;35(4):385-393. doi:10.3233/RNN-160704
75. Abdelgadir, IS, Gordon, MA, & Akobeng A. Melatonin for the management of sleep problems in children with neurodevelopmental disorders: A systematic review and meta-analysis. Drug Ther (NY). 2018;103(12):1163-1167.
76. Poeggeler B, Reiter RJ, Tan DX, Chen LD, Manchester LC. Melatonin, hydroxyl radical-mediated oxidative damage, and aging: a hypothesis. J Pineal Res. 1993;14(4):151-168.
77. Mayo JC, Sainz RM, Tan D-X, et al. Anti-inflammatory actions of melatonin and its metabolites, N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) and N1-acetyl-5-methoxykynuramine (AMK), in macrophages. J Neuroimmunol. 2005;165(1-2):139-149. doi:10.1016/j.jneuroim.2005.05.002
78. Carrillo-Vico A, Reiter RJ, Lardone PJ, et al. The modulatory role of melatonin on immune responsiveness. Curr Opin Investig Drugs. 2006;7(5):423-431.
79. Bielli A, Scioli MG, Mazzaglia D, Doldo E, Orlandi A. Antioxidants and vascular health. Life Sci. 2015;143:209-216. doi:10.1016/j.lfs.2015.11.012
80. Lundmark PO, Pandi-Perumal SR, Srinivasan V, Cardinali DP, Rosenstein RE. Melatonin in the eye: implications for glaucoma. Exp Eye Res. 2007;84(6):1021-1030. doi:10.1016/j.exer.2006.10.018
81. Kozirog M, Poliwczak AR, Duchnowicz P, Koter-Michalak M, Sikora J, Broncel M. Melatonin treatment improves blood pressure, lipid profile, and parameters of oxidative stress in patients with metabolic syndrome. J Pineal Res. 2011;50(3):261-266. doi:10.1111/j.1600-079X.2010.00835.x
82. Mayo JC, Sainz RM, Antoli I, Herrera F, Martin V, Rodriguez C. Melatonin regulation of antioxidant enzyme gene expression. Cell Mol Life Sci. 2002;59(10):1706-1713.

 1-800-758-8746
1-800-758-8746